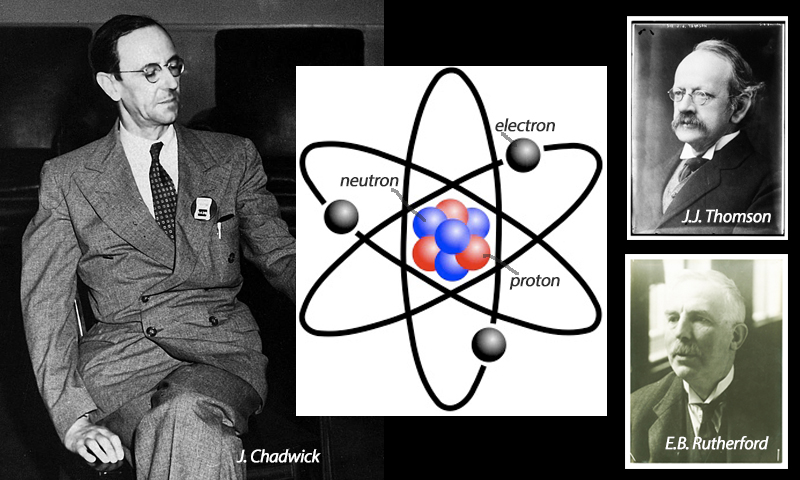
Neutrons
Mathematician and physicist Sir James Chadwick (1891-1974) from Cheshire discovered the third type of sub-atomic particle, namely neutrons, for which he received the 1935 Nobel Prize in Physics. Negatively charged electrons had been found in 1897 by Sir J.J. Thomson (1856-1940) and positively charged protons in 1919 by Lord Ernest Rutherford (1871-1937). Chadwick was both a student and, subsequently, research associate of Rutherford and, for over a decade, resolutely explored Rutherford’s suggestion that there must be a neutral, uncharged particle as well, to make up the known atomic number and weight.
The technology was not sufficiently advanced to prove this until 1932, by which time cloud chambers and particle accelerators had been developed at the University of Cambridge’s Cavendish Laboratory ~ home at one time or another to all three of Thomson, Rutherford and Chadwick.
Chadwick was able to set up an experiment to bombard the element beryllium with alpha particles and break it apart to observe the scattered protons’ velocity. He calculated the properties of neutral rays sailing on regardless through various types of barrier and concluded that they were the neutrons, with atomic mass almost identical to that of protons. This was a key moment in the new field of nuclear science, for good and bad.
(Images: photos Public Domain & Rutherford-Bohr atomic model: nshepheard at Flickr.com / CC BY-SA 2.0)
